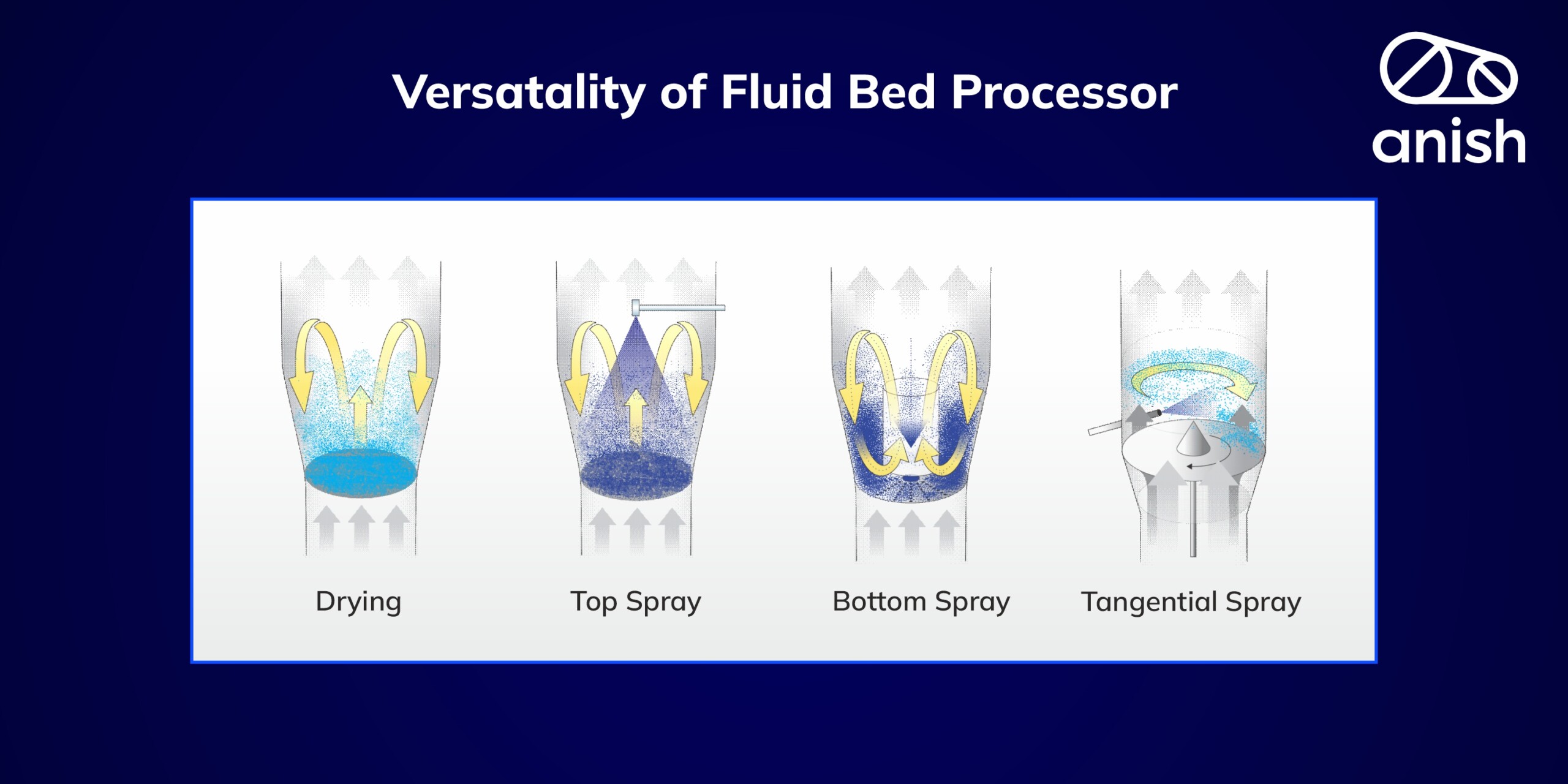
The word “Granulated” is derived from the Latin ” granulatum “, meaning grain. The granulation process is, thus, the pharmaceutical process of widening or enlarging finer powder particles. In this process, small particles gathered together with the help of a binder form a permeant bulky granule. This process is widely used in the mining, detergent, and agriculture industries. All industries use this particle enlargement technique to reduce dust formation, facilitate ease of handling, and improve material effectiveness.
The tablet press invention was a huge breakthrough in the development of pharmaceutical granulation technology. The demand for granulation properties was boosted when high-speed capsule filling and tablet compression machines were introduced with automated controls. These high-speed machines require an even flow of feeding materials through the hopper to produce quality dosage forms.
The granulation method converts powders into free-flowing, dust-free granules that are easy to compress. The blend uniformity or content uniformity of the low-dose-containing products can be achieved using this technology. The combination of the process and formulation controls the desired characteristics of granules. The characteristics of the particles developed after granulation depends on the following factors:
- Particle size of the drug and excipients.
- Type, concentration, and volume of binder or solvents.
- Granulation process time.
- Type of granulator used for manufacturing.
- Drying rate (temperature and time).
Granulation procedures are of two types: wet granulation and dry granulation. Wet granulation utilizes liquid as a medium for binding particles, and dry granulation does not need a liquid binder. The procedure selection requires thorough knowledge of the physicochemical properties of the drug, excipients, release properties, etc. The pharmaceutical granulation process is widely used for manufacturing tablets, capsules, modified-release spherical granules, and granules as sprinkle dosage forms.
The granulation process is carried out to:
- Improve the density of the powdered material.
- Improve the flow property of the blend and reduce the weight variation problem between tablets and capsules.
- Increase the uniformity of the Active Pharmaceutical Ingredient (API) throughout the dosage form.
- Manufacture dust-free granules.
- Improve the hardness of tablets.
- Improve the appearance of the product.
There are four mechanisms which are involved in the development of granules from powdered or fine raw materials.
Wetting and Nucleation: Wetting is the process of uniformly distributing a liquid binder onto the surface of the powdered or fine raw materials. It is achieved by adding a liquid binder, often a solution or suspension, to the dry powder. It is crucial in granulation as it helps to enhance the adhesion between particles, improving their cohesiveness and facilitating the subsequent agglomeration or binding steps.
Nucleation forms small seed particles, known as nuclei, from the wetted powder mass. These nuclei act as the starting points for further growth and agglomeration, leading to the formation of granules. Nucleation is a critical step as it determines the size and characteristics of the granules. The control of nucleation can be achieved by adjusting the binder properties, such as binder concentration, viscosity, and drying rate.
Coalescence & Growth: Coalescence refers to the process of two or more smaller particles coming together to form a larger particle. It typically occurs when the particles in a granulation process collide and adhere to each other, forming larger agglomerates or granules. Coalescence is influenced by various factors such as particle size, shape, surface properties, and the presence of binding agents or liquid bridges that promote particle sticking. It leads to the formation of larger and more cohesive granules with improved flow properties, compressibility, and content uniformity.
In wet granulation, growth primarily involves the deposition of liquid binder onto the particles, causing them to adhere and increase in size. The liquid binder can also facilitate coalescence between particles, leading to further growth. Both coalescence and growth are important processes in granulation as they contribute to the formation of well-formed and functional granules. The extent of coalescence and growth can be controlled by adjusting various process parameters such as binder concentration, mixing intensity, granulation time, and equipment design to achieve the desired granule characteristics.
Consolidation: In wet granulation processes, consolidation is achieved by applying pressure during the drying phase. The wet granules are typically dried using the fluid bed. The applied pressure during drying aids in compacting the granules, removes moisture and enhances inter-particulate bonding.
Consolidation is crucial for the final properties of granules. It improves their flowability, compressibility, and stability during subsequent processing steps, such as tablet compression or capsule filling. Proper consolidation ensures the granules can withstand mechanical stresses without breaking or deforming, producing high-quality final dosage forms.
Attrition or Breakage: Attrition or breakage in granulation refers to the process by which granules undergo fracture or fragmentation, resulting in smaller particles or fines. It can occur during various stages of the granulation process, including particle size reduction, mixing, transportation, or compression. Attrition or breakage can have both intended and unintended consequences in granulation processes, depending on the desired particle size distribution and the specific application.
Intended Attrition: In specific granulation processes, intentional attrition or breakage is desired to achieve a particular particle size distribution or control the final product’s release characteristics.
Unintended Attrition: Unintended attrition or breakage can occur due to various factors such as excessive mechanical forces, abrasive interactions, or poor handling practices. It can lead to the generation of fines or smaller particles, which may affect the final product’s quality attributes, such as content uniformity, flowability, or compressibility.
The above-mentioned mechanisms can happen concurrently in all wet granulation techniques, like high shear granulation (RMG), spray drying, and top spray granulation. Whereas some granulating mechanisms may dominate in specific granulation processes, the wetting process influences top spray granulation in fluid bed processors. In high shear granulation, mechanical redispersion of binder is done by impellers and choppers, reducing the powder blend’s wetting. On the other hand, granule consolidation is a more noticeable phenomenon in high-shear mixing than in top spray granulation. All these mechanisms control the final granule size distribution, granule structure, and porosity resulting from the process and ultimately contribute to the quality of the granulated product. Regulatory bodies are promoting the Quality by Design (QbD) concept for manufacturing quality drug products worldwide.
anish pharma is one of the leading granulation machine manufacturers in India. Our granulation line is built with the expertise of more than three decades and with the help of seasoned pharmaceutical industry experts to have the best-in-class technology and deliver top-notch results. To know more, connect with us.
 Search
Search