In today’s pharmaceutical industry, granulation is vital in ensuring the production of high-quality and consistent granules for various Oral Solid Dosage forms (OSD), including tablets and capsules. Achieving robust formulations that meet stringent standards requires a delicate balance of process parameters and equipment. anish pharma, a renowned name among pharma equipment manufacturers, offers a range of granulation machines, including the Rapid Mixer Granulator (RMG), Fluid Bed Dryer (FBD), Dry & Wet Mills, Blenders and accessories. This blog will delve into the critical aspects of granulation, highlighting the significance of robust formulations and efficient technology transfer.
Raw material loading by vacuum transfer system: The journey commences with the vacuum transfer system, efficiently filling the RMG bowl with the raw material. This system ensures a clean and seamless transfer of ingredients.
Dry and wet mixing: The anish RMG automates both dry and wet mixing processes. This automation enhances efficiency and consistency in granule formation. Precise dry and wet mixing times ensure the thorough and uniform distribution of the Active Pharmaceutical Ingredient (API) and other excipients throughout the powder blend.
Binder addition: anish RMG facilitates precise binder addition with larger spray widths while maintaining small drop sizes. It promotes faster nucleation, a crucial granule growth and coalescence element during the kneading phase.
Impeller and chopper design: The RMG bowl is equipped with impellers and chopper blades. anish pharma’s design optimises powder flow patterns and shear rates, ensuring thorough mixing and granulation. The combined action of the impeller and chopper is crucial for ensuring the overall quality and consistency of the granules. The impeller and chopper design is crucial for consistent drug content and physical properties in granule production, ensuring efficacy, safety, and uniformity in final pharmaceutical products.
Optimising impeller speed for robust formulation: During product development and technology transfer, selecting the right impeller speed is pivotal. anish recommends starting with higher impeller speeds until “Roping” behaviour is observed. Roping occurs when material from the powder bed’s bottom is forced up the vessel wall and tumbles back towards the centre of the bowl. This behaviour indicates good rotation and vertical turnover.
Calculating the scale-up factor from small-scale RMG to large-capacity RMG involves considering tip speed and bowl diameter ratios.
Endpoint control for granulation: Determining the endpoint of granulation involves measuring specific power consumption or impeller torque as the wet mass granulates. This data reflects changes in powder rheology during granulation. Typically, power consumption increases as a liquid is added, indicating improved cohesion, a significant consideration for fluid bed granulator users.
anish RMG displays torque and power consumption on the screen, simplifying process standardisation while maintaining other parameters consistently.
Efficient material transfer to co-mill: After achieving the granulation endpoint, the RMG initiates material transfer to the wet co-mill. Standardising the impeller speed for discharge is essential for seamless transfer, a factor highly regarded by pharma equipment manufacturers.
The particle size of wet granules depends on chopper speed, co-mill sieve, and co-mill speed.
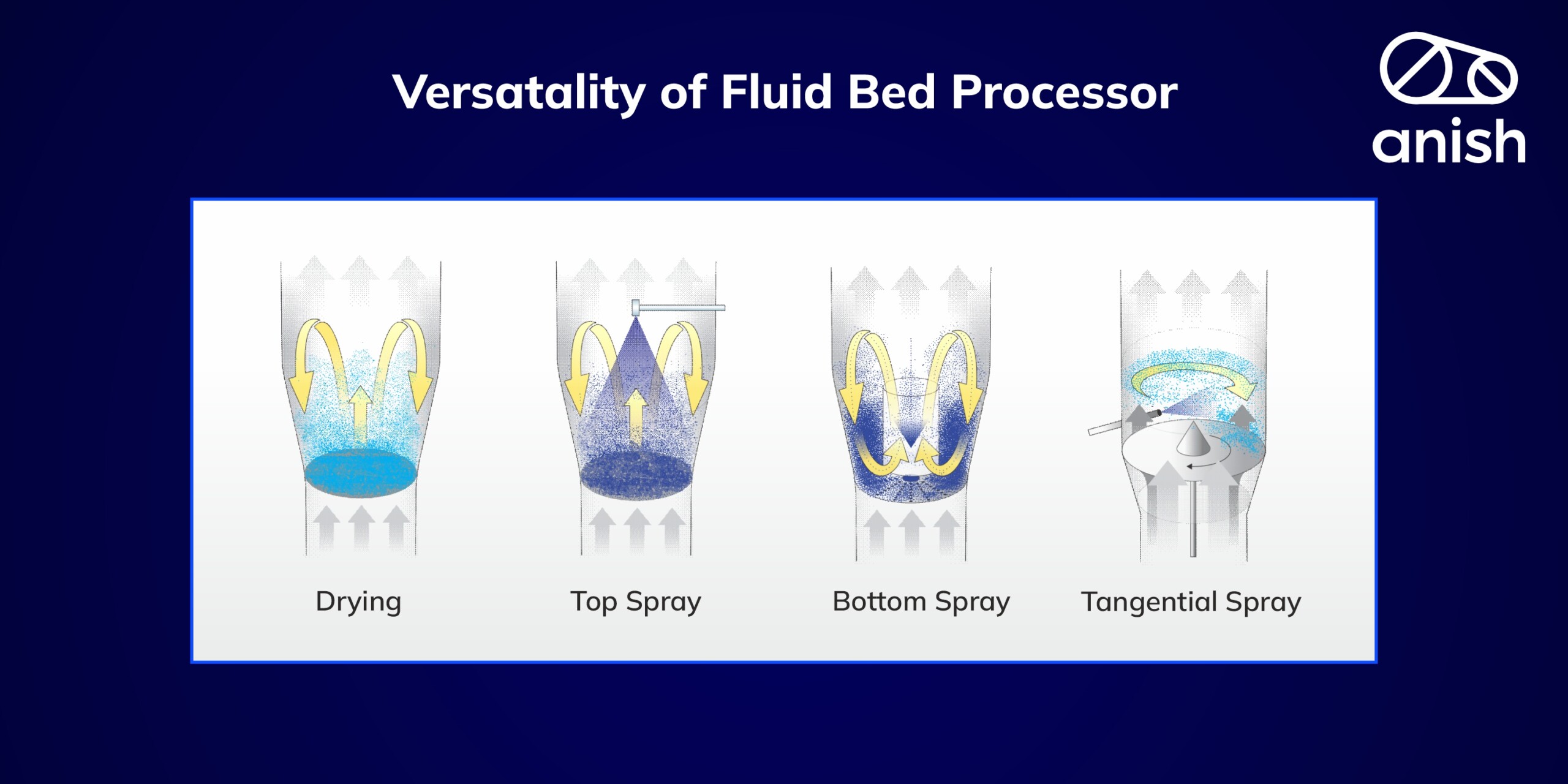
Seamless drying in anish fluid bed dryer (FBD): By carefully controlling the drying conditions, anish fluid bed dryers help maintain the integrity of the granules. Over-drying or uneven drying can lead to changes in the physical and chemical properties of the granules, affecting their flowability, compressibility, and other critical characteristics needed for further processing.
Dry Milling: After drying, the granules are vacuum transferred to the deck sifter. Oversize granules are passed through the dry co-mill for further refinement. Dry milling can contribute to the stability of the formulation by creating a uniform particle size distribution that minimises the risk of segregation or uneven drug distribution within the final product. This step can enhance the pharmaceutical formulation’s long-term stability and shelf life.
Blending: Blender systems in pharmaceutical granulation are essential for achieving the desired properties of the final product, such as flowability, compressibility, and uniformity. The choice of a blender and the specific parameters used during the blending process can significantly affect the quality and characteristics of the final granules.
Advanced Control System: Advanced control systems allow for real-time monitoring of critical process parameters, such as temperature, moisture content, and granule size distribution. Real-time monitoring enables quick detection of deviations from the set parameters, allowing immediate corrective actions to maintain product quality and consistency. Ensures the granulation process adheres to strict regulatory standards, such as Good Manufacturing Practices (GMP). Compliance with these standards is crucial for ensuring pharmaceutical products’ safety, efficacy, and quality and meeting regulatory requirements set by authorities such as the FDA.
Scalable Granulation process: Scalability ensures that the granulation process can be seamlessly transitioned from small-scale development to large-scale commercial production. It enables pharmaceutical manufacturers to optimise production efficiency, minimise production costs, and meet the growing demand for pharmaceutical products. Regulatory authorities, such as the FDA, require pharmaceutical manufacturers to demonstrate that the manufacturing processes are scalable without compromising product quality and safety.
anish pharma manufactures 1L to 1800L linear scalable granulation equipment.
In the world of pharmaceuticals, achieving robust granulation forms the foundation of quality formulations. anish pharma’s Close Loop Granulation Lines, equipped with state-of-the-art RMGs & Fluid Beds, simplify the granulation process while ensuring precision and consistency. These machines handle a wide range of feed powders and liquid binders, making them the ideal choice for pharmaceutical manufacturers.
anish pharma’s commitment to quality and innovation has established it as a trusted name in the industry. With anish pharma, you can elevate your granulation process to new heights of efficiency and reliability. To learn more about the anish granulation lines and other pharmaceutical equipment, get in touch with our expert technical team.
 Search
Search