Granulation, a fundamental process in pharmaceutical manufacturing, involves particle enlargement through agglomeration. This technique is indispensable for the production of tablets, capsules, and taste-masked powders. At anish pharma, we understand the significance of granulation in pharmaceutical formulation. In this article, we delve into the world of granulation techniques, emphasising the importance of incorporating advanced technology to achieve robust formulations.
The Granulation Process
Granulation initiates with the initial dry mixing of powder ingredients, including the Active Pharmaceutical Ingredient (API), aiming to create a homogeneous powder mixture. This preparatory step ensures that the API is uniformly distributed throughout the formulation. It’s a critical starting point, and our commitment to precision at anish pharma is reflected in this crucial phase.
The objectives of granulation are multifaceted.
- Enhancing API uniformity: The process helps achieve uniform distribution of the API, ensuring consistent dosage in the final product.
- Improving blend density: By increasing the blend density, we reduce the volume occupied per unit weight, leading to more efficient storage and shipment. Granules typically range in size from 0.2 to 4.0 mm, but they are often produced in an intermediary size range of 0.2 to 0.5 mm. These intermediary granules can be conveniently packed in a dosage form or mixed with other excipients before tablet compaction or capsule filling.
- Facilitating metering: Precise dosing and volumetric dispensing are made possible through well-granulated formulations.
- Reducing dust and hazards: Effective granulation minimises dust during the process, mitigating toxic exposure and process-related hazards.
- Enhancing appearance: The visual appeal of the final product is significantly improved, creating a positive impression in the market.
Desirable granule characteristics:
The ideal granules possess several key characteristics:
- Spherical shape: Spherical granules enhance flow properties, ensuring a smooth manufacturing process.
- Narrow particle size distribution: A tight distribution promotes content uniformity and facilitates volumetric dispensing.
- Adequate fines: The presence of fines helps fill void spaces between granules, enhancing compaction and compression characteristics.
- Appropriate moisture and hardness: Optimal moisture levels and hardness are crucial to prevent granule breakage and dust formation during the process.
Factors influencing granule properties:
Several factors influence the properties of granules post-granulation:
- Particle size: Both the drug and excipients’ particle sizes play a significant role.
- Binder and solvents: The type, concentration, and volume of binders and solvents are critical.
- Granulation time: The duration of the granulation process affects the final product.
- Type of granulator: Different granulators yield different results.
- Drying rate: The temperature and time of drying are important considerations.
Processes in granulation:
The granulation process can be divided into three key rate processes:
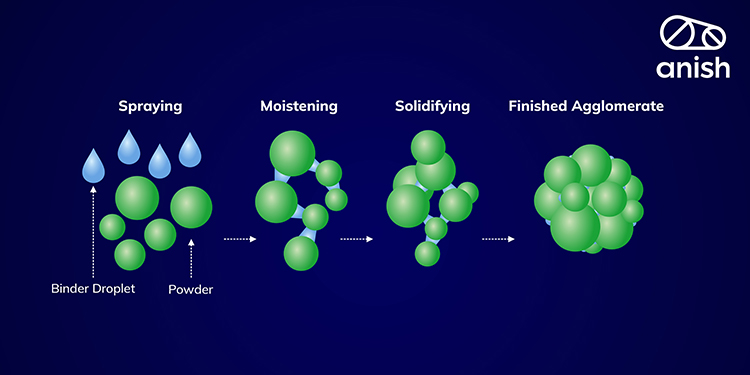
1. Wetting, Nucleation, and Binder Distribution:
In the initial stage of granulation, known as wetting, the focus is on achieving the even distribution of the binder within the powder mixture to form granule nuclei. This process is pivotal as it sets the foundation for the entire granulation process. Proper wetting is paramount because it directly impacts the size distribution of these nuclei.
Effective wetting occurs when each binder droplet thoroughly immerses itself into the powder bed, enveloping individual particles to create well-formed granule nuclei. Inadequate wetting of powder by binder droplets or slow imbibition can result in larger, less uniform wet agglomerates on the powder bed.
Sometimes, within the powder bed, there are shear forces generated that can break up these lumps of wet material, aiding in the further distribution of the liquid binder. Therefore, the wetting phase is a critical step where precise control is required to ensure that the binder is uniformly distributed, and the nuclei formation results in a narrow size distribution.
2. Consolidation and growth:
Once granule nuclei are formed, the next stage involves consolidation and growth. Granule nuclei consolidate by colliding with other granules and experiencing varying degrees of resistance to deformation. The extent of consolidation is influenced by factors such as the intensity of agitation within the granulator and the inherent resistance of the granules to deformation.
Granules created from fine powders or with binders that are viscous in nature tend to resist deformation upon impact, leading to slower consolidation. Conversely, granules produced from coarse powders deform significantly upon impact and quickly reach their minimum porosity. Granule consolidation plays a pivotal role in determining the final granule porosity, which in turn affects several other granule properties.
Moreover, when two granules collide, they may adhere together, forming a single, larger granule through a process known as coalescence. For successful coalescence, it’s essential that the energy generated during the collision is absorbed, preventing the granules from rebounding. Additionally, a strong bond must form at the contact point between the colliding granules. The presence of liquid at the surface of the granule is critical for facilitating growth through coalescence, and the coalescence rate is highly sensitive to the liquid content.
3. Attrition and breakage:
The final stage of the granulation process involves the fate of dry granules. They may either break into fragments or attrit into fine powder. The extent to which this occurs is determined by granule properties, particularly fracture toughness and porosity. Granules that possess high fracture toughness and have fewer and smaller flaws are more resistant to attrition.
Understanding these three stages of granulation – wetting and binder distribution, consolidation and growth, and attrition and breakage – is essential in tailoring the granulation process to achieve the desired properties in the final granules. This knowledge allows for precise control over the process, ensuring the production of high-quality pharmaceutical formulations. The choice of the Granulators will depend upon the final size, density and scale of operation required.
The table provides information on various machines, highlighting their distinct advantages and product specifications
| Equipment | Particle size (mm) | Density | Scale of operation | Applications | Machines available with anish |
| Tumbling Granulators, Disc Granulators | 0.5 to 20 | Moderate | 500 kg/hr to 5000 kg/hr | Food, Chemicals | – |
| Mixer Granulators, Continuous High Shear Granulators, | 0.1 to 2 | Low to High | Upto 50 Ton/hr | Pharmaceutical, Chemicals, Food | – |
| Batch High Shear Granulators | 0.1 to 2 | High | 1 kg to 1000 kg /batch | Pharmaceutical | anish mixer granulator |
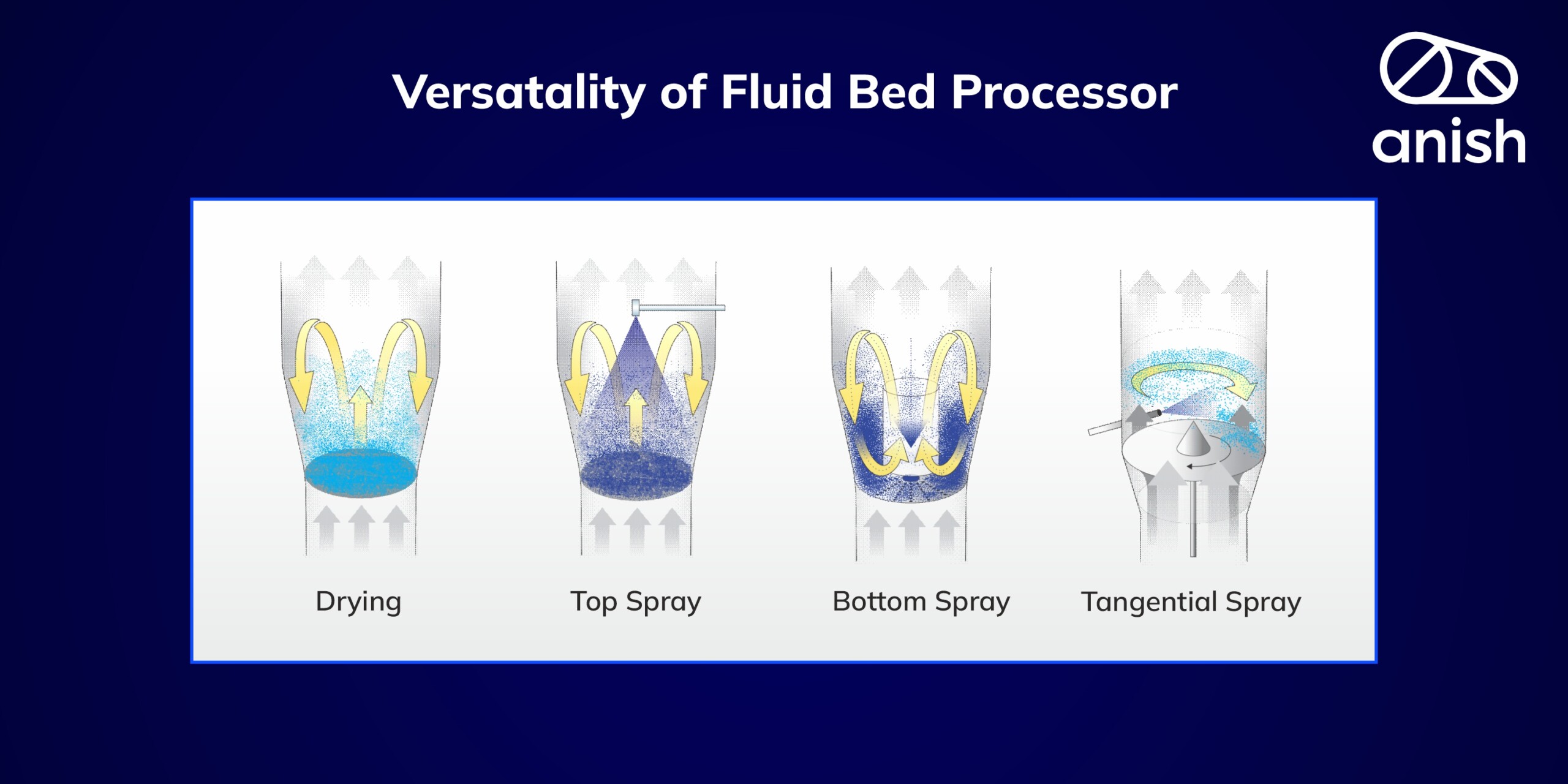
| Fluidized Bed – Top Spray | 0.1 to 2 | Low (agglomerated) | 1 kg to 1000 kg/batch | Pharmaceutical, Food, Nutraceutical, Chemical | anish fluid bed – top spray |
| Fluidized Bed – Bottom Spray (Wurster) | 0.1 to 2 | Moderate (layered) | 1 kg to 1000 kg/batch | Pharmaceutical, Food, Nutraceutical, Chemical | anish fluid bed – bottom spray |
| Centrifugal Granulators (Tangential Rotor) | 0.3 to 3 | Moderate to High | Upto 300 kg/batch | Pharmaceutical, & Nutraceutical | anish fluid bed -tangential rotors |
| Pressure Compaction Roll Compactor | 0.7 to 2 | High | Upto 300 kg/hour | Pharmaceutical | anish roll compactor |
| Pressure Compaction through Extruder, and Spheronizer | 0.6 to 1.2 | High | Upto 300 kg/hour | Pharmaceutical, Food, Nutraceutical | anish extruder & anish spheronizer |
Choosing the right equipment:
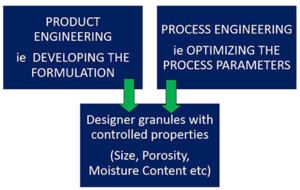
Selecting the appropriate granulation equipment is vital. Factors like manufacturing process risk assessments, literature survey-based drug formulation knowledge, and the effect of moisture, shear, and temperature on the drug substance, final granule size, density, and scale of operation play a significant role in this decision-making process. At anish pharma, we combine manufacturing process risk assessments, in-depth drug formulation knowledge, and an understanding of the impact of moisture, shear, and temperature on the drug substance to make informed choices.
Granulation is the cornerstone of pharmaceutical formulation, and its success hinges on understanding processes and selecting the right equipment. anish pharma continually advances our granulation techniques to achieve robust formulations that meet the most stringent specifications. Our commitment to excellence ensures that pharmaceutical dosage forms produced under our guidance are of the highest quality and efficacy. Visit www.anishpharma.com to learn more about anish’s equipment.
 Search
Search